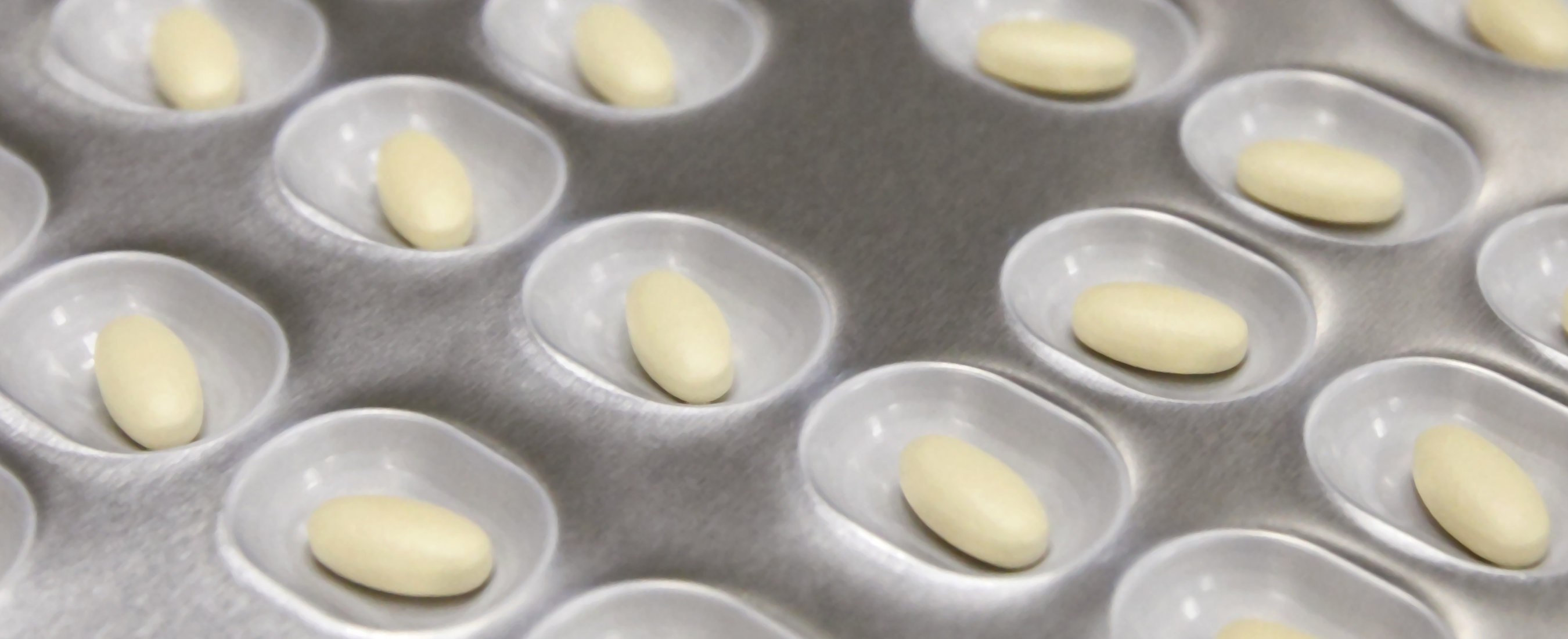
Krka’s innovative generic products have been tested in everyday clinical practice and are accessible to a wide range of users. Our medicines are used to treat the most common diseases of our time, helping more than 100 million people in over 70 countries every day. Krka produces high-quality, safe and effective medicines. Part of our program is devoted to animal health.
Product search
In our commitment to healthy living we offer a broad range of high-quality prescription pharmaceuticals, non-prescription products and animal health products.
Krka’s innovative products

250
active ingredients

150
single-pill combinations

1100
brands

1000
pharmaceutical forms